Which of the Following Statements Correctly Describe a Redox Reaction
Is a exothermic reaction. It produces 25 ATP for every NADH this is oxidized.

Redox Reactions Examples Types Applications Balancing
It involves the redox reactions of the electron transport.

. Which of the following statements correctly describes the oxidation-reduction reaction shown below. C 6 H 1 2 O 6 H 2 S O 4 c o n c 6 C 6 H 2 O. Select all the statements that correctly describe the following redox reaction which is given as a molecular equation.
NAD NADH H A- B Reactants NAD NADH and H combine to form products A. B Free radicals are produced as the result of homolytic cleavage of a. During a redox reaction the locations of electrons do not change just the energy level of the electrons the total number of electrons on the products and the.
A redox reaction can easily be explained as. 7Which particles are gained and lost during a redox reaction. It is a series of redox reactions Requires mitochondria The method yeast use to produce ATP Pyruvate Oxidation is one of the steps Glycolysis is one of the steps.
Mg s 2HCI aq Mgc2aH2 g Check all that apply. 1 Oxidation and reduction occur simultaneously. But when an element is reduced it gains electrons.
Aelectrons B neutrons C protons D ions 8When a redox reaction occurs there must be a transfer of A double-replacement. The temporary storage of cellular energy. H is the oxidizing.
H 2 S O d i l Z n C O 3 Z n 2 C O 2 S O 4 2 H 2 O. A change in oxidation number B. For the following redox reaction indicate which reactants are oxidized and reduced.
Therefore it is a redox reaction. Which of the following is incorrect concerning oxidative phosphorylation. The formation of ions 17.
A Free radicals carry a single unpaired electron. When an element is oxidized it loses electrons. Which of the following statements correctly describes a redox reaction.
C Oxidation-reduction reaction or redox reaction is defined as the reaction in which oxidation. Which of the following statements isare correct about the following reactions. For B - B 2 a O 1 2 H 2 SO 2 4 B a 2 SO 2 4 H 2 O 1 2 Here no change of oxidation state takes place.
The breakdown of glucose in cells. The term redox is a short form of reduction-oxidation. A redox reaction always involves A.
Which statement correctly describes a redox reaction. Select all the statements that correctly describe the following redox reaction which is represented by the molecular equation below. ClO_3- aq 6Fe_2aq 6Haq rightarrow Cl- aq 6Fe3 aq 3H_2O l.
2 Oxidation occurs before reduction. Mg s 2HCl aq MgCl2 aq H2 g a Cl- is reduced to Cl22-. A spontaneous redox reaction occurs which converts chemical energy to electrical energy.
Oxidation occurs at the negative electrode anode. During a redox reaction electrons are lost the products have fewer electrons than the reactants. A change in phase C.
Place the following steps for balancing a redox reaction that occurs in acidic solution in the correct order. Third option is the correct one. In the reaction Cl2 2Br aq.
Which of the following statements correctly describe a redox reaction. HCl aq Ni s KCr 0 aq KCl aq CrCl aq Nici. Select all the statements that correctly describe the following redox reaction which is given as a molecular equation.
Select all that apply-Multiple select question-Electrons always move from an atom that attracts them less strongly-Electrons always move toward an atom that is electron poor or electron deficient-This type of reaction involves the transfer of electrons. B a reaction that results in the release of heat or light. Redox reactions are oxidation-reduction chemical reactions in which the reactants undergo a change in their oxidation states.
Select ALL that are correct. Electricity is conducted by the. Separate the unbalanced reaction into half-reactions.
Which of the following statements regarding free radicals is false. 3 Oxidation occurs after reduction. Forming a bond by sharing electrons.
This is the basis of redox reactions. The transfer of protons D.
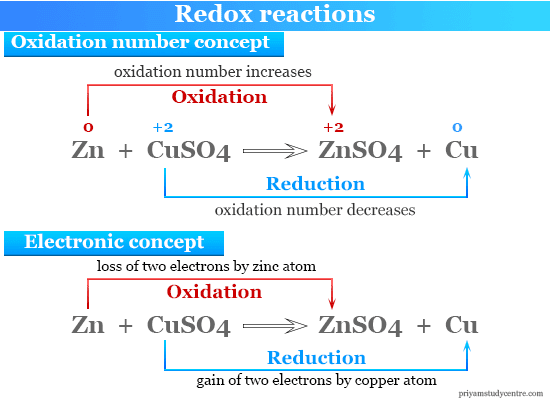
Redox Reactions Definition Types Examples Application
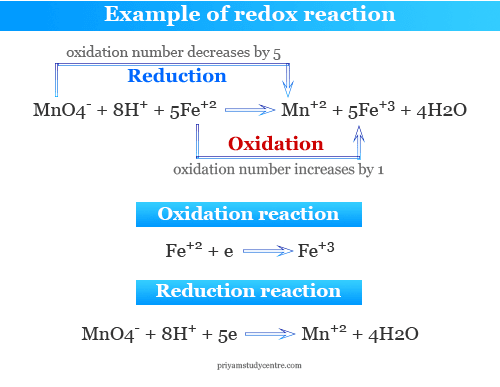
Redox Reactions Definition Types Examples Application
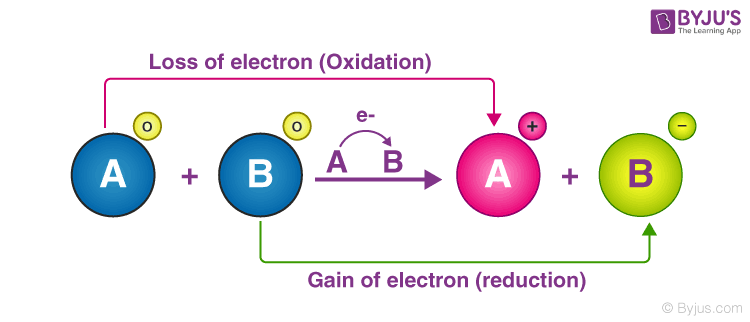
0 Response to "Which of the Following Statements Correctly Describe a Redox Reaction"
Post a Comment